Definition and Chemical Structure of Acid Dyes
by : Rahul Sharma
Acid Dyes:
Acid dyes are highly water soluble, and have better light fastness than basic dyes. The textile acid dyes are effective for protein fibers such as silk, wool, nylon and modified acrylics. They contain sulphonic acid groups, which are usually present as sodium sulphonate salts. These increase solubility in water, and give the dye molecules a negative charge. In an acid solution, the -NH2 functionalities of the fibers are protonated to give a positive charge: -NH3+. This charge interacts with the negative dye charge, allowing the formation of iconic interactions. As well as this, Van-der-Waals bonds, diploar bonds and hydrogen bonds are formed between dyes and fibers. As a group, acid dyes can be diveded into two sub-groups: Acid-leveling and Acid-milling.
Chemical Structure of Acid Dyes:
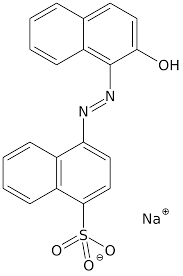
These dyes are normally very complex in structure but have large aromatic molecules, having a sulphonyl or amino group which make them soluble in water. Most of the acid dyes belongs to following three main structural molecules.
- Anthraquinon type
- Azo dye type
- Triphenylmethane type
