Cyanine dyes:- (Part 1)
by : ---
Cyanine dyes:-
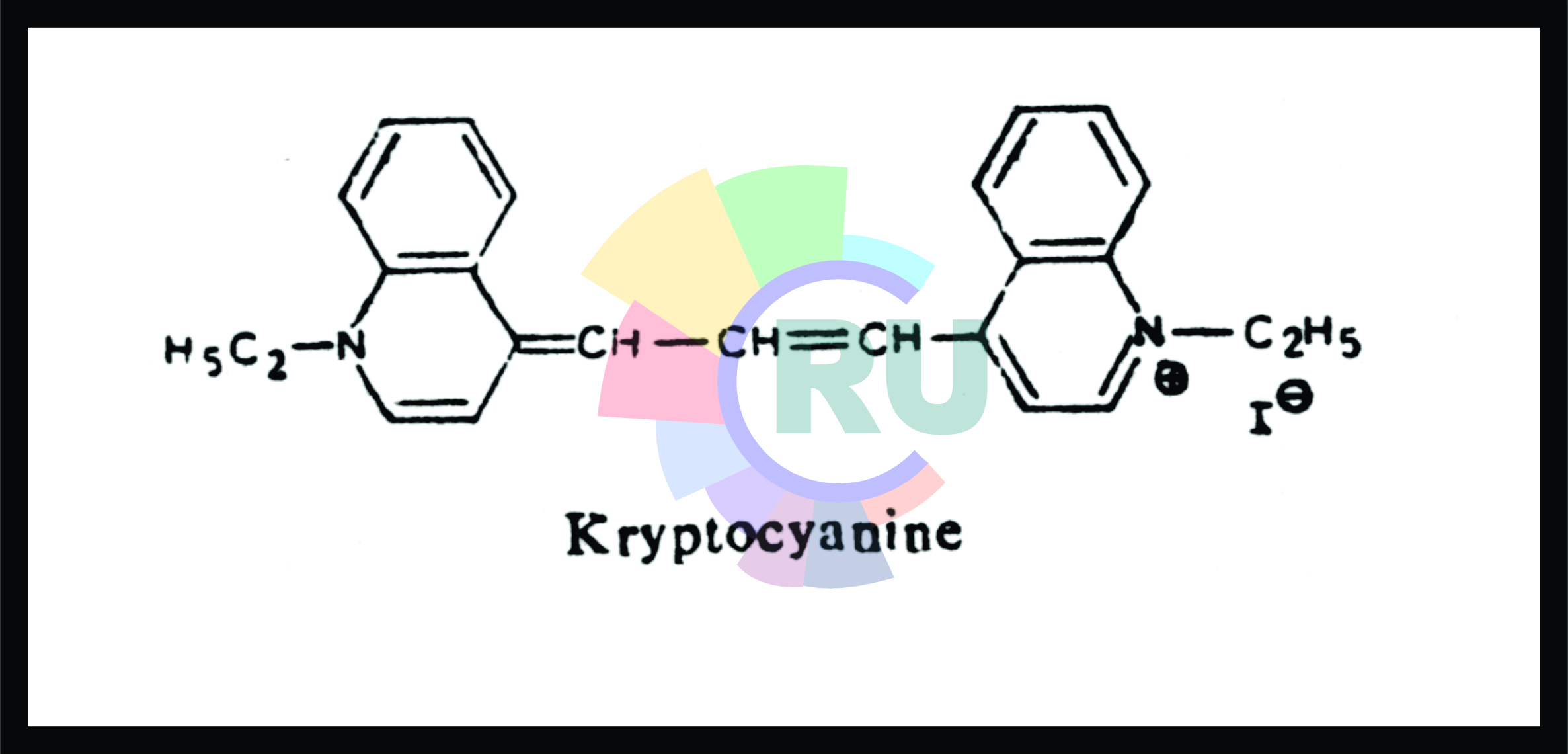
Cyanine dyes have two nitrogen containing ring systems in one of these the nitrogen is quaternary and in another, it is tertiary, these two nitrogen atoms are linked by a conjugated chain of an uneven number of carbon atoms, such as —CH= and —CH = CH —CH =, etc. or similar chains containing nitrogen atoms such as =CH—N=N— etc. THe heterocyclic nitrogen containing system such as quinoline, pyridine indole, benzothiazole, etc. are employed in their synthesis.
There are various classes of classes of cyanine dyes mainly depending upon the positions of teterocyclic system attached to each other and the nuber and type of linkages of carbon chain or similar chain between two nitrogen atoms. For simplicity, an example is given below. Kryptocyanine is a cyanine dye derived from quinoline derivatives, both the heterocylic nitrogen systems being quinoling. It belongs to a substass carbocyanine as the two rings are joined by the linkage —CH=CH—CH=, and since the positions of attachment are 4,4', (the ring systems are linked through their 4 positions) this carbocyanine dye falls under Kryptocyanines.
