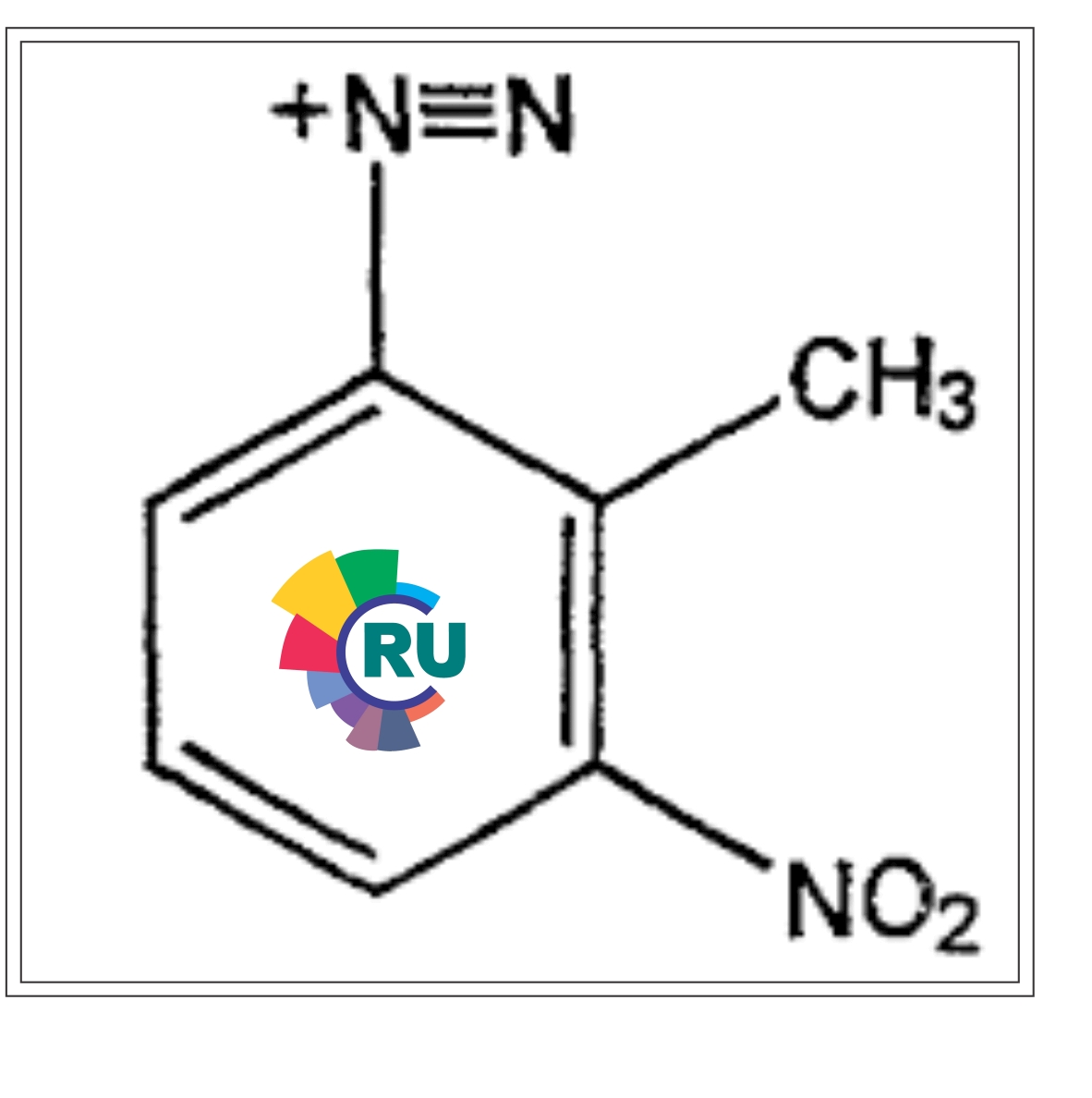
Diazinium colour formers
by : --
One obvious colour formation potentially of use in pressure and thermally sensitive systems is the snythesis of azo compounds. Over the past ten years the Ricoh Co. of Japan has described a number of patents related to the use of microencapsulated coupling compqonent is exemplified [30] by Naphtol AS (C.I. Azonic coupling Component 2). Both the coupler and a catalyst are encapsulated in a low melting present as solution in a second coating and this is in contact with the coating containing the encapsulated coupler. Image formation takes place by exposing the composited film to u.v. radiation under a transparent document. Decomposition of the exposed diazonium salt takes place subsequent develpment by heat causes release of the encapsulated coupler which can react immediately with the un-exposed diazonium salt [31]. A catalyst commonly used is stearamide, C17H35CONH2, or tis N-alkylated (C1-C5) derivatives [32] which can be separately encapsulated and used in a single layer containing both diazo and coupler, neither of which is encapsulated [33]. Although simple diazonium compounds stablilised as their zinc chloridek double salts can be used, more compex derivative are usually emplyed. Since aqueous solutions are more difficult to encapsulate, (water-in-oil emulsions are generally less stable) only the coupler and / or catalyst is encapsulated. In at least one intance, however, [34] both components are separately encapsulated in a thermoplastic resin and the capsules dispersed in a single coating layer
Currently the diazonium colour formers are not of commercial importance
