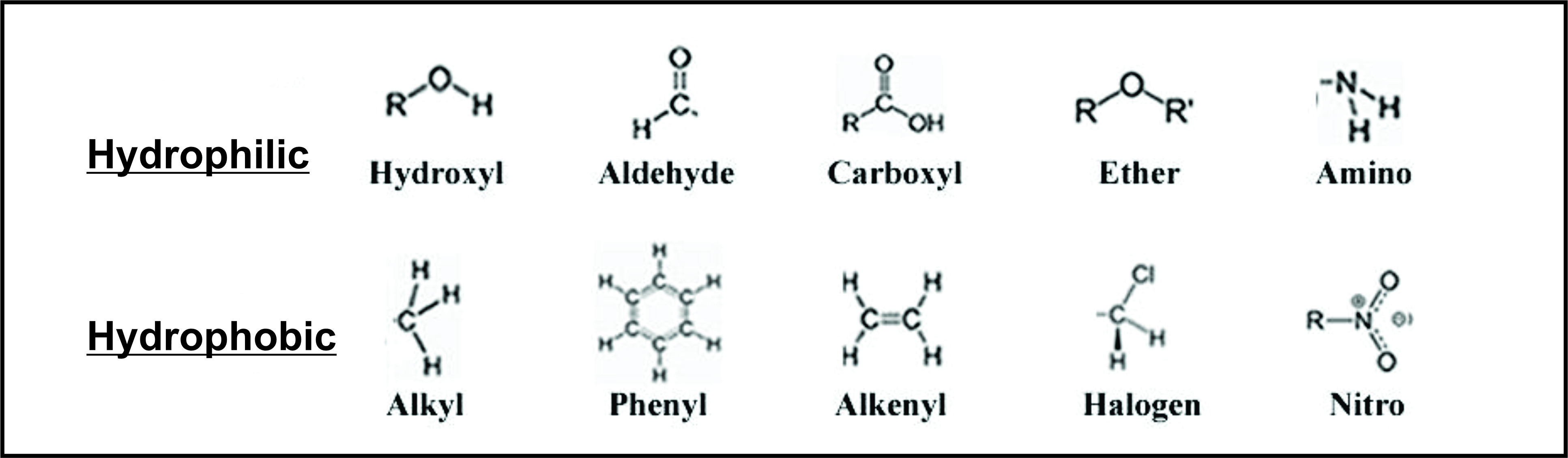
Hydrophobic Rest Hydrophilic Group:-
by : ---
Hydrophobic Rest Hydrophilic Group:-
The hydrophobic character of the hydrocarbon chain increases as the chain gets longer. Within the hydrophobic regions of individual molecules are van der Waals forces which hold those regions together. Alkane-derived alcohols become more viscous as the length of their chains increases, because forces within the molecule become more and more exaggerated. Solubility in water decreases as molecule size grows. The hydrophilic hydroxyl group of the molecule forms hydrogen bonds with other neighbouring hydroxyl groups. These hydrogen bonds are stronger than normal van der Waals forces, and they influence the melting and boiling points of alcohols. The melting and boiling points of alcohols, therefore, are significantly higher than those of alkanes.
