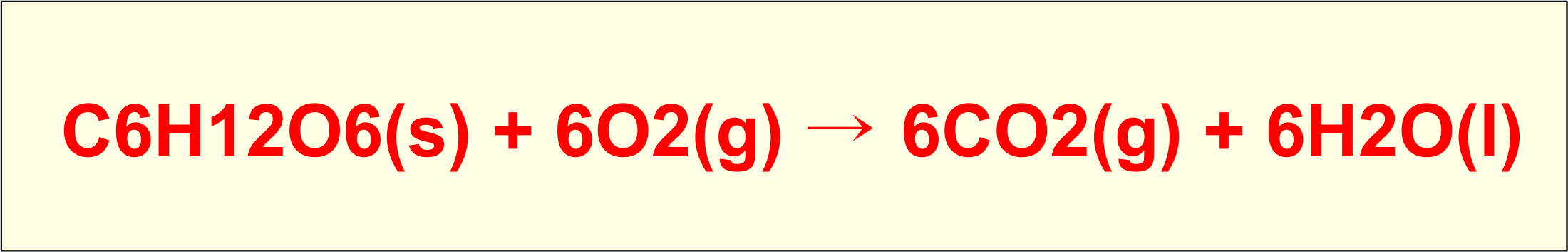
Oxidising Agents: Explained Completely
by : Rahul Sharma
Oxidising Agents:
Oxidizing agent is a substance that has the ability to oxidize other substances in other words to accept their electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combustion, many explosives, and organic redox reactions involve atom-transfer reactions.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
